Top Pharma News in March 2025

This month’s healthcare news highlights public health governance updates involving the Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC), as well as trade restrictions affecting medical technology advancements. These events carry notable implications for the health and pharmaceutical sectors. To support informed decision-making, Fullintel Hub provides expert insights, in-depth analysis, and timely updates on significant healthcare and policy changes, ensuring stakeholders remain current with evolving developments.
March’s Top Stories
Healthcare Highlights Include the White House Withdrawal of Trump’s CDC Nominee, the Resignation of the FDA’s Top Lawyer, and China’s Ban on Illumina Sequencers Following Trump’s Recent Tariffs

The top three developments shaping this month’s healthcare news are:
- The White House withdraws Trump’s nomination for CDC director hours before the confirmation hearing.
- FDA Chief Counsel Hilary Perkins resigns two days into her role.
- China bans imports of Illumina’s gene sequencers following new U.S. tariffs.
Developments in public health leadership and trade policy influence March’s media landscape, drawing public and political attention. The White House’s withdrawal of Dr. Dave Weldon’s CDC nomination due to Senate opposition generates the highest volume of coverage and triggers the most social media engagement, largely expressing concern over political interference in public health leadership. This is followed by the resignation of FDA Chief Counsel Hilary Perkins, which sparks debate surrounding vaccine mandates and abortion policies. China’s ban on Illumina’s gene sequencers, enacted in response to new U.S. tariffs, receives mixed reactions. While some express concern over trade impacts, analysts highlight potential growth opportunities for Illumina due to global demand for gene-sequencing technologies.

A Breakdown of Recent Trending Stories:
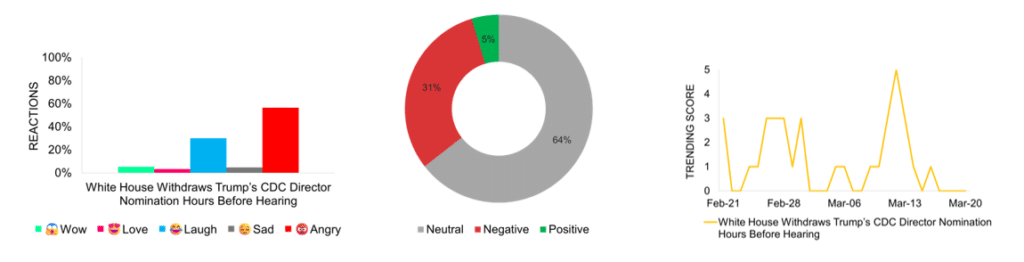
White House Withdraws Trump’s CDC Director Nomination Hours Before Hearing
The White House withdraws Dr. Dave Weldon’s nomination as CDC director just hours before his confirmation hearing, citing insufficient Senate support. Weldon, a vocal vaccine skeptic, faces opposition from Republican senators, including Susan Collins and Bill Cassidy, with Health Secretary Robert F. Kennedy Jr. also questioning his readiness for the role. This move raises concerns about the administration’s approach to public health leadership, with fears of regulatory delays. Media attention peaks on March 13, the day of the announcement, with largely neutral coverage. While some sources support Weldon’s policy views, others point to institutional instability and transparency issues. Social media reactions reflect frustration with the nomination and its withdrawal. The event amplifies concerns about the administration’s role in shaping public health policy, particularly as the CDC continues to operate without a permanent director.
FDA’s Top Lawyer Quits Following Senate Criticism
Hilary Perkins resigns from her role as FDA Chief Counsel after two days, following criticism from Senator Josh Hawley over her previous support for abortion rights and vaccine mandates. As a career government lawyer, Perkins has previously defended policies under both President Trump and Biden. Her resignation reflects the impact of political pressure on civil servants and raises concerns about the strength of legal positions in the government. The public response, especially from conservatives, highlights divisions on healthcare matters. The controversy fuels backlash, with critics questioning Perkins’ qualifications and judgment, deepening debates over vaccine mandates and abortion policies. The story gains traction towards the end of February, peaking on March 13 when the resignation is announced. Most media coverage is mostly neutral, with some negative reports expressing concerns about the potential impact of Perkins’ resignation on the FDA’s legal and healthcare initiatives. Social media responses are mixed, with notable ‘Anger’ and ‘Laugh’ reactions, indicating public frustration with political interference. Approximately 10% of reactions express surprise (‘Wow’), reflecting the unexpected nature of the resignation. The FDA has not yet named a successor, leaving uncertainty about who will assume the position.
China Imposes Import Ban on Illumina Gene Sequencers Amid Trump Tariffs
On March 4, China bans imports of Illumina’s gene sequencers, following its February 4 decision to place the company on its “unreliable entity” list in response to a new 10% U.S. tariff. The move presents operational challenges for Illumina, although the company remains active in China through the sale of reagents and consumables not covered by the ban. Chinese competitors BGI Genomics and MGI Tech benefit, with share prices rising. Meanwhile, proposed caps on NIH funding could reduce Illumina’s revenue by 1–2%. The majority of media coverage is neutral, though some criticism emerges over the impact of government tensions on scientific progress. Analysts maintain cautious optimism about Illumina’s long-term prospects, citing growing demand for gene-sequencing technologies. Social media reactions vary: ‘Laugh’ reactions highlight the irony of Illumina’s previous lobbying against Chinese competitors, while ‘Anger’ and ‘Sad’ responses stem from concerns over NIH funding cuts and their implications for innovation. Illumina CEO Jacob Thaysen now faces the task of steering the company through an uncertain geopolitical and economic environment.
Angela Dwyer is an award-winning, media measurement expert who helps brand improve business results through data-driven, actionable insights.