Top Pharma News in October 2025

This month’s healthcare and pharmaceutical coverage underscores the growing tensions between medical innovation, public policy, and patient safety. From the FDA’s shift in COVID-19 vaccine guidance to controversial political backing of unproven autism research, and the rise of AI-powered diagnostic tools such as smart stethoscopes, the industry is navigating a rapidly changing environment. These developments highlight ongoing debates around scientific integrity, healthcare access, and the responsible use of emerging technologies. The Fullintel Hub delivers curated insights to help stakeholders respond effectively to the evolving healthcare narrative.
October’s Top Stories:
COVID Shot Age Limits, Autism-Tylenol Controversy, and AI Stethoscope Innovation Lead September’s Pharma Buzz
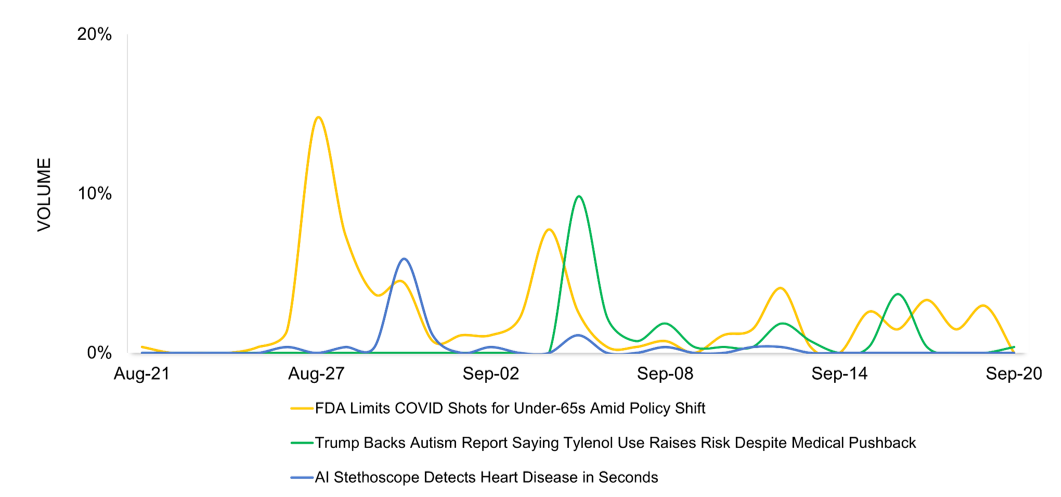
Three key developments shape healthcare media coverage in October:
- FDA Limits COVID Shots for Under-65s Amid Policy Shift
- Trump Backs Autism Report Saying Tylenol Use Raises Risk Despite Medical Pushback
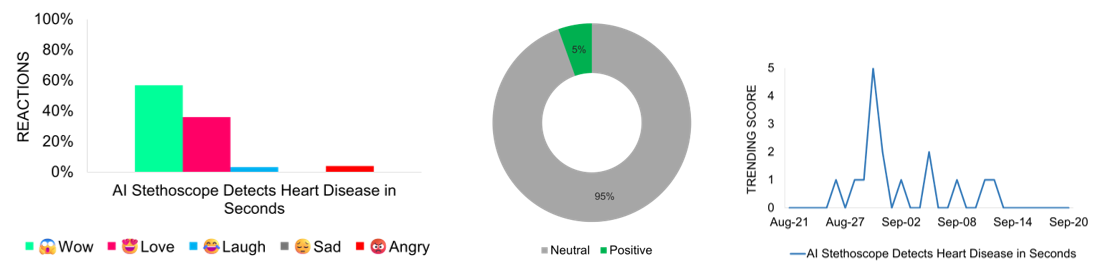
- AI Stethoscope Detects Heart Disease in Seconds
The Trump administration’s decision to limit FDA approval of updated COVID vaccines to seniors and high-risk patients under 65, and to end routine access for healthy children and pregnant women, stands out as the top story of the month, garnering the highest engagement. A planned report linking Tylenol use in pregnancy to autism follows, facing strong medical rejection. Another notable story highlights an AI-powered stethoscope that detects heart disease in 15 seconds, improving early diagnosis despite some false positives.
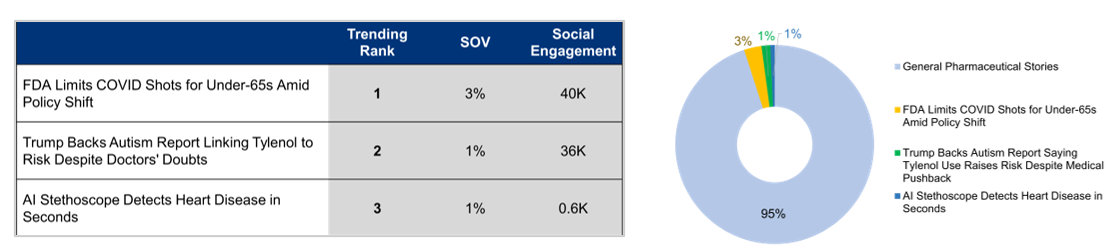
A Closer Look at October’s Top Pharma Headlines:
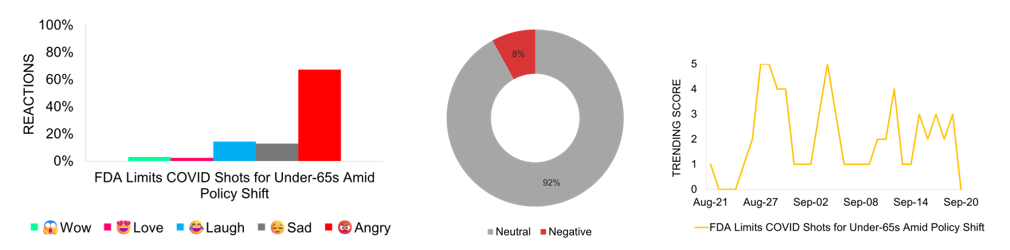
FDA Limits COVID Shots for Under-65s Amid Policy Shift
The Trump administration, led by Health Secretary Robert F. Kennedy Jr., restricts FDA approval of updated COVID-19 vaccines to seniors and people under 65 with high-risk conditions. This change ends routine access for healthy children and pregnant women. Officials emphasize that vaccines remain available to everyone, but experts point to practical barriers such as off-label prescribing and insurance coverage. Former Surgeon General Jerome Adams and other critics argue the decision contradicts earlier commitments to universal access. Medical associations continue to recommend broad vaccination, warning of possible winter surges. Media reports are largely neutral, centering on policy shifts and access concerns. Online discussions show frustration, confusion, and skepticism, reflecting a broader uncertainty about the federal approach.
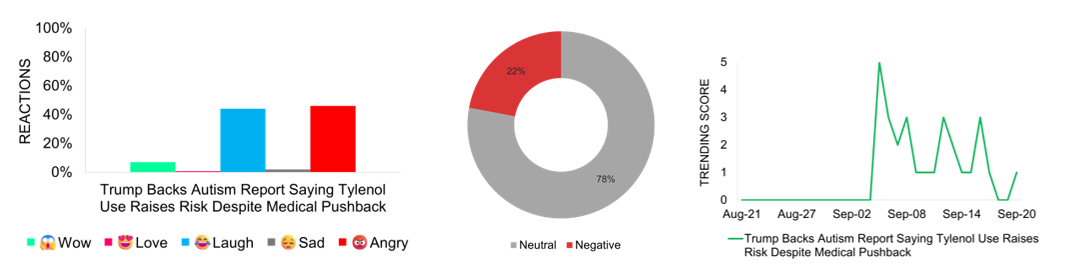
Trump Backs Autism Report Linking Tylenol Use To Risk Despite Medical Pushback
Doctors and major medical organizations strongly oppose the Trump administration’s plan, led by Health Secretary Robert F. Kennedy Jr., to release a report claiming a link between Tylenol use during pregnancy and autism. Medical experts emphasize that no credible evidence supports this connection and warn that it may create unnecessary fear among expectant mothers. Analysts note the move reflects increasing political influence over reproductive health and could undermine confidence in safe treatment options. The American College of Obstetricians and Gynecologists maintains that Tylenol remains safe when used as directed. Media coverage peaks on September 5 and continues in subsequent days, showing a neutral to negative tone overall. Online reactions, particularly from doctors and patient advocates, mix anger with disbelief and humor, underscoring deep concern over the politicization of medical science.
AI Stethoscope Detects Heart Disease in Seconds
A new collaborative study by Imperial College London and Eko Health highlights the potential of an AI-powered stethoscope to detect heart failure, atrial fibrillation, and heart valve disease during routine GP visits. Tested on more than 12,000 patients, the device analyzes heart sounds and ECG data in just 15 seconds, identifying atrial fibrillation 3.5 times more often than standard care. While researchers acknowledge the possibility of false positives, the technology significantly enhances early detection and facilitates faster clinical intervention. Findings were presented at the European Society of Cardiology Congress and published in BMJ Open. Media coverage peaks on August 30 and 31, exhibiting a 95% neutral tone and a balanced focus on both the study’s promise and limitations. Online reactions are largely positive, with expressions of excitement and curiosity about the AI tool’s potential to enhance cardiovascular care.
Coverage in the following month continued exploring the intersection of healthcare innovation and government policy, including new debates around AI-driven cancer research, drug pricing reforms, and fertility access.
Katie Michel is the Insights Manager at Fullintel, where she specializes in media analysis and reporting, transforming complex data into actionable insights for clients. With a background in Marketing Communications Research from Boston University, she has contributed to academic projects, including work on Dr. Michael Elasmar's textbook, "An Introduction to Self-Report Measurement."





